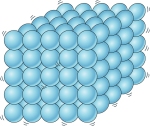
SOLID
- Solids have very low kinetic energy (the energy of movement)
- The particles vibrate in place
- Solids have a definite shape and volume (they cannot be compressed)
- The particles are very close together
- The particle attraction is very strong
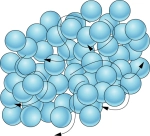
LIQUID
- Liquids have high kinetic energy
- The particles are able to move past each other
- Liquids take the shape of the container they are in (they do not have a definite shape)
- Liquids have a definite volume (they cannot be compressed into a smaller space)
- There are spaces between the particles
- The particles have low particle attraction
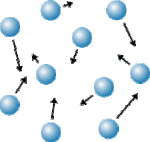
GAS
- Gases have very high kinetic energy
- Gas particles move freely
- The particles fill the space they are in (they do not have a definite shape or volume)
- Gases CAN be compressed into smaller spaces
- There are very large spaces between the particles
- The particles have very low particle attraction
*** Kinetic energy increases as you move from SOLIDS to GASES
*** Particle attraction decreases as you move from SOLIDS to GASES

No comments:
Post a Comment