Chemistry! Hooray!

Tuesday 7 June 2016
Chapter 1 Links for Review
1.1 Crossword
https://sciencesource.pearsoncanada.ca/puzzles/puzzle_01_1/
1.2 Crossword
https://sciencesource.pearsoncanada.ca/puzzles/puzzle_01_2/
1.3 Crossword
https://sciencesource.pearsoncanada.ca/puzzles/puzzle_01_3/
1.1 Quiz
https://sciencesource.pearsoncanada.ca/quizzes/quiz_01_1SLjG2.htm
1.2 Quiz
https://sciencesource.pearsoncanada.ca/quizzes/quiz_01_2vRCiM.htm
1.3 Quiz
https://sciencesource.pearsoncanada.ca/quizzes/quiz_01_35YRu2.htm
Read about food chains
http://www.vtaide.com/png/foodchains.htm
Food Chain Examples
http://www.coolantarctica.com/Antarctica%20fact%20file/wildlife/whales/food-web.php
Brain Pop Video on Food Chains
https://www.brainpop.com/science/ecologyandbehavior/foodchains/
Brain Pop Food Chain Game
https://www.brainpop.com/games/foodchaingame/
Desert Food Web Example
http://www.vtaide.com/png/desertBiomes.htm
Boreal Forest Food Web Example
http://www.vtaide.com/png/taiga.htm
Temperate Rain Forest Example
http://www.vtaide.com/png/temperateBiomes.htm
Practice Food Webs
Meadow
http://www.harcourtschool.com/activity/food/meadow_activity.html
Arctic
http://www.harcourtschool.com/activity/food/arctic_activity.html
Pond
http://www.harcourtschool.com/activity/food/pond_activity.html
Study Guide for Grade 7 Chapter 1 Test
Key Terms
□ abiotic
□ bacteria
□ biotic
□ carnivores
□ chlorophyll
□ community
□ consumers
□ decomposers
□ detritivores
□ ecosystem
□ food chain
□ habitat
□ herbivores
□ nutrients
□ omnivores
□ organic matter
□ oxygen
□ photosynthesis
□ populations
□ predator
□ prey
□ producers
□ scavengers
□ species
· Examples of biotic – biotic, abiotic – biotic, and biotic – abiotic interactions
· The process of photosynthesis
· The five basic needs of living things
· The roles of producers
· Predator-prey relationships
· Food chains and food webs
Friday 27 May 2016
Chapter 10 Links for Review
10.1 Quiz
https://sciencesource.pearsoncanada.ca/quizzes/quiz_10_1paqKt.htm
10.2 Quiz
https://sciencesource.pearsoncanada.ca/quizzes/quiz_10_2KLylM.htm
10.3 Quiz
https://sciencesource.pearsoncanada.ca/quizzes/quiz_10_3YEEdN.htm
10.4 Quiz
https://sciencesource.pearsoncanada.ca/quizzes/quiz_10_4fY7Rd.htm
10.1 Crossword
https://sciencesource.pearsoncanada.ca/puzzles/puzzle_10_1/
10.2 Crossword
https://sciencesource.pearsoncanada.ca/puzzles/puzzle_10_2/
10.3 Crossword
https://sciencesource.pearsoncanada.ca/puzzles/puzzle_10_3/
10.4 Crossword
https://sciencesource.pearsoncanada.ca/puzzles/puzzle_10_4/
Chapter 10 Review Quiz
http://wps.pearsoned.ca/ca_school_ontarioscience_7-8/102/26141/6692106.cw/content/index.html
Chapter 10 Matching Terms
https://sciencesource.pearsoncanada.ca/resources/gr7_matchquiz_ch10/
Chapter 10 Labeling Practice
https://sciencesource.pearsoncanada.ca/resources/gr7_labelquiz_ch10/
Convection Example Video
https://sciencesource.pearsoncanada.ca/resources/battlingbottles.php
Heat Radiation Animation
https://sciencesource.pearsoncanada.ca/resources/radiation.swf
Conduction, Convection, Radiation Animation
http://www.wisc-online.com/objects/heattransfer/
Pop-Can Implosion Video
https://sciencesource.pearsoncanada.ca/pgs/video.php?id=12
Adding Heat to Particles Activity/Animation
https://sciencesource.pearsoncanada.ca/resources/slg.swf
Forms of Energy Matching
https://sciencesource.pearsoncanada.ca/resources/03_forms.swf
Thermal Radiation Face Matching
http://spaceplace.nasa.gov/ir-matching/en/
Energy Transformation Activity
http://www.phschool.com/webcodes10/index.cfm?fuseaction=home.gotoWebCode&wcprefix=cad&wcsuffix=1020
Information About Energy Transformations
http://www.energykids.eu/energy-transform
Types of Energy PowerPoint
https://sciencesource.pearsoncanada.ca/pgs/resource.php?found=slideshow_gr7_pg283_energyforms.ppt&plevel=7
Energy Transformation PowerPoint
https://sciencesource.pearsoncanada.ca/pgs/resource.php?found=slideshow_gr7_pg284_transform.ppt&plevel=7
How a Thermometer Works
http://www.energyquest.ca.gov/how_it_works/thermometer.html
What is an Expansion Joint
http://en.wikipedia.org/wiki/Expansion_joint
Beat the Heat! Game
http://spaceplace.nasa.gov/beat-the-heat/en/
Infrared Image Game
http://spaceplace.nasa.gov/ir-photo-album/en/
Wednesday 25 May 2016
Chapter 10 Test Study Guide
Chapter 10
Test Study Guide
Review the
following:
Textbook Section
10.1
1. All 10 forms of energy
2. How energy can be transformed from on type of energy to another; e.g., a candle burns chemical energy and it is changed to light energy and thermal energy.
Review Section 10.2
1. The definitions of temperature, heat, and thermal energy. Understand the difference between these three.
Review Section
10.3
1. Review how solids, liquids and gases behave when heated or cooled. (Expansion and Contraction)
2. Understand how we apply our knowledge of solids liquids and gases expanding and contracting in our daily lives; e.g., leaving space for air at the top of sealed bottles of liquids. (Review package worksheets)
10.4
1. Review the three forms of thermal energy transfer (conduction, convection, and radiation) and how they work.
2. Understand how we apply our knowledge of thermal energy transfer; e.g., wearing light clothes on hot days.
Key Terms
1. Review how solids, liquids and gases behave when heated or cooled. (Expansion and Contraction)
2. Understand how we apply our knowledge of solids liquids and gases expanding and contracting in our daily lives; e.g., leaving space for air at the top of sealed bottles of liquids. (Review package worksheets)
10.4
1. Review the three forms of thermal energy transfer (conduction, convection, and radiation) and how they work.
2. Understand how we apply our knowledge of thermal energy transfer; e.g., wearing light clothes on hot days.
Key Terms
□
chemical energy
□
conduction
□
convection
□
convection current
□
elastic energy
□
electrical energy
□
energy
□ energy transformation
□ fluids
□
gravitational energy
□
heat
□
infrared waves
□
light energy
□
magnetic energy
□
mechanical energy
□
nuclear energy
□
particle theory of matter
□ thermometer
□
radiation
□
sound energy
□
temperature
□ thermal
energy
Chapter 10 Notes
Here are some notes for section 10.2 and 10.3
Temperature – The
average speed of the particles in a substance (we use a thermometer to take the
temperature)
Heat – Is the
thermal energy transferred from an area of higher temperature to lower
temperature
Conduction
· The
transfer of heat through a solid or between a solid and another solid, a
liquid, or a gas that is in contact with it
· Conduction
can also occur from liquid to solid and gas to solid.
· Conduction
always occurs from a warmer region to a cooler region.
· Conduction
occurs when warmer particles bump into cooler particles making them vibrate
faster.
· Metals
are better heat conductors than non-metals because free electrons carry heat
quickly through the material.
Convection
· Convection -
The transfer of thermal energy by moving particles in fluids
· As
the particles of fluids (gases and liquids) are heated, they move faster and
expand; this expansion makes the fluids less dense so they rise
· When
warmer fluids rise, cooler fluids move downwards; this creates a “convection
current”
· The
convection current continues in a pot of soup because the warmer soup particles
in a pot cool when they make contact with the air and then sink. These
particles are heated again once they meet the bottom of the pot.
· Convection
currents help heat your home through your furnace heating system.
Radiation
· Radiant
energy is the transfer of energy by invisible waves given off by the
energy source
· These
invisible waves are called infrared waves (a type of electromagnetic wave from
the sun)
· Infrared
waves are given off by all heat sources, including you
· Heat
is radiant energy from the sun that reaches your skin
· Radiant
energy warms up objects when the waves of radiant energy come into contact with
matter making the particles vibrate faster
Answers to Conduction and Radiation Worksheets
Answers to
Conduction Worksheet
1. On a cold winter day, why would an iron post in a park feel much colder to the touch than a wooden bench?
1. On a cold winter day, why would an iron post in a park feel much colder to the touch than a wooden bench?
Iron is a better heat conductor than wood. The
iron will conduct the heat away from your hand faster than wood; this makes
your hand feel colder when you touch iron
2. Potatoes cook from the outside in.
a) Why
does a small potato cook faster than a large potato?
Smaller potatoes have fewer particles, so heat takes
less time to reach the centre.
b) Why
does sticking a metal skewer through the middle of a potato make it cook
faster?
Heat will be conducted through the skewer into the
centre of the potato and it will cook from the inside out.
Answers to Radiation Worksheet
1) Radiation waves are absorbed by the person in front
of you so you do not feel the heat.
2) White clothing reflects infrared radiation and
black clothing will absorb this radiation.
3) The shiny suits will reflect the strong heat
radiation from the sun so the astronauts do not overheat.
4) Insulation is a poor heat conductor and it contains
glass pieces to reflect heat back into your home.
5) In summer, heat is reflected out into the
environment and in winter, heat is reflected back into your home.
Thursday 19 May 2016
Answers to the Expansion and Contraction Worksheets
Solids Expanding and
Contracting in our Daily Lives
- Why is it
important to place gaps at regular intervals in sidewalks?
In the summer the sidewalk expands and in the
winter it contracts. The gaps allow space for the expansion and contraction so
the sidewalk doesn’t crack.
- Concrete and
steel expand at almost the same rate. Explain why this is important in the
construction of tall buildings.
If they did not expand and contract at the same
rate, there would be many cracks in the building and it might fall down.
Gases Expanding and
Contracting in our Daily Lives
- Explain how a
hot air balloon is lifted from the ground.
When the gas particles inside the hot air
balloon are heated, they expand and some escape from the bottom of the balloon.
This causes there to be fewer air particles inside the balloon then outside the
balloon. The less dense hot air will float on more dense cold air so the
balloon rises.
c) Once in the air, the burner is turned off
and the balloon drifts along with the wind. What will eventually happen to the
air inside the balloon?
Cooler air will enter the balloon and the air
inside will become more dense. The balloon will begin to go down.
Liquids Expanding and
Contracting in our Daily Lives
- A bowl of hot
soup was left on the table to cool. After a few minutes, the amount of
soup in the bowl appeared to have decreased? Why?
The soup may have cooled down and there seems
to be less soup because the particles become closer together (contract) and
become denser.
- When
manufacturers pack liquids into bottles and jars, they leave a small space
at the top before putting on the lids. Why?
The space allows for the liquid to expand
without causing the bottle to break.
- Mercury
expands and contracts faster than alcohol. Which liquids would be better
in a thermometer?
Mercury will tell the temperature faster, but
it will also contract faster so it will drop more quickly making it harder to
read the temperature. Also, mercury is poisonous!
Monday 16 May 2016
Monday 4 April 2016
Chapter 8 Test Study Guide
Chapter 8 Test Study Guide
Chapter 8 Key Terms
□ alloy
□ concentrated solution
□ concentration
□ dilute solution
□ insoluble
□ saturated solution
□ saturation point
□ solubility
□ solute
□ solvent
□ supersaturated solution
□ unsaturated solution
□ alloy
□ concentrated solution
□ concentration
□ dilute solution
□ insoluble
□ saturated solution
□ saturation point
□ solubility
□ solute
□ solvent
□ supersaturated solution
□ unsaturated solution
□ universal solvent
□ rate of dissolving
Separation Methods
□ filtration
□ magnetism
□ evaporation
□ flotation
□ distillation
□ paper chromatography
□ sifting
□ settling
□ flotation
□ distillation
□ paper chromatography
□ sifting
□ settling
□ sorting
□ separation funnel
□ picking out with tongs
Areas to Review
- What factors affect the rate of dissolving? How do these factors affect the rate of dissolving?
- How can a solution be made more concentrated or less concentrated?
- What are the 2 different meanings of the term “solubility”? (There is a qualitative and quantitative definition)
- Calculate the concentration of of a solution in grams/100 mL and describe it as a concentrated or dilute solution
- Complete the check and reflect questions in Chapter 8. They will be taken up in class.
Remember! you can access the textbook online from home!
Chapter 8 Review Activities
Here are some review activities and crosswords to help you study.
Section 8.1 Quiz
https://sciencesource.pearsoncanada.ca/quizzes/quiz_08_1XJ85P.htm
Section 8.2 Quiz
https://sciencesource.pearsoncanada.ca/quizzes/quiz_08_2jTk44.htm
Section 8.3 Quiz
https://sciencesource.pearsoncanada.ca/quizzes/quiz_08_3pvz04.htm
Chapter 8.1 Crossword
http://www.sciencesource.ca/puzzles/puzzle_08_1/
Chapter 8.2 Crossword
http://www.sciencesource.ca/puzzles/puzzle_08_2/
Chapter 8.3 Crossword
http://www.sciencesource.ca/puzzles/puzzle_08_3/
Chapter 8 Matching Quiz
http://www.sciencesource.ca/resources/gr7_matchquiz_ch08/
Chapter 8 Labeling Quiz
http://www.sciencesource.ca/resources/gr7_labelquiz_ch08/
Separating Mixtures Labeling
http://www.sciencesource.ca/resources/05_sep.swf
A good explanation of chromatography (be sure to click "show labels")
http://www.sciencesource.ca/resources/gr7_label_page228.swf
A good explanation of dissolving
http://www.sciencesource.ca/resources/gr7_label_page221.swf
Separating Mixtures
http://www.bbc.co.uk/bitesize/ks3/science/chemical_material_behaviour/compounds_mixtures/revision/9/
Wednesday 30 March 2016
Filtration Worksheets Due on Monday April 4th
Please submit all completed worksheets for the filtration project on Monday April 4th.
Monday 7 March 2016
Rate of Dissolving Lab Due Date
The rate of dissolving lab is due for all classes on:
Friday March 11th, 2016
I will collect the labs from all students by lunch so please bring your completed lab to Your period 3 class.
Wednesday 2 March 2016
Dear Parents
Thank you for following my blog!
The TDSB has asked teachers to begin using Google Classroom. This is an app that allows students to download and upload documents from the site as well as complete quizzes, watch videos, etc.
I will continue updating the blog with assignment due dates, test dates, as well as study guides; however, it is important that every student is accessing the Google Classroom for additional materials.
I have included information in previous posts to help students access the Google Classroom. If your child has forgotten his or her username/password, I will happily help them upon request.
Please email me at anthony.meles@tdsb.on.ca with any questions or concerns.
Thank you,
Anthony Meles
Tuesday 23 February 2016
Link to google classroom
Follow the link below to Google Classroom and use your username and password from school to log in.
https://classroom.google.com/u/1/h
Tuesday 16 February 2016
Google Classroom Access and Class Codes
To access Google Classroom, you will first need to enter Academic Workspace.
To enter Academic Workspace
1. Google TDSB Academic Workspace and select first link
2. Enter you login and password you use at school; remember your user name is your student number
To enter Google Classroom
1. Click on the Google Apps tab and select Classroom
To join your class
1. Click on the + sign beside your name in the top right hand corner of the page
2. Select Join class
3. Enter your class code:
7A: ymg2d4
7B: xewfpl
7C: sd7jjcm
7D: nrh2k02
7E; jik5rg
Congratulations! Your in!
Thursday 11 February 2016
At this point, some classes are brainstorming filter designs. Please follow these steps when brainstorming:
1. Read about water filter design by exploring the links provided below
2. Consider what would be a good design for a water filter?
3. Using the brainstorming worksheet provided in class, draw a filter you believe would work well to separate a mechanical mixture
4. Remember to add detailed notes to your diagram that explains the rationale behind the materials and design you used
5. You will also need to include estimated measurements for your design; remember that the filter must fit inside a 30 cubic centimeter box
Links to explore:
http://www.ourtravellifestyle.com/tips-and-planning/travel-tips/science-fun-experiments-travel-kids/
Wednesday 10 February 2016
Section 8.1 Notes
Solutions: Concentration and Solubility
Solutions
- are
homogeneous mixtures
- have the same
appearance throughout
Alloys – a special name for solid solutions
(e.g., steel)
Solute – the substance
that dissolves
Solvent – the substance
which does the dissolving
E.g., in sugar and water, the water is the solvent and the sugar is the
solute
Water – The Universal Solvent
Water is able to dissolve many different solids, liquids and gases
Not all substances are soluble in water; e.g., fat is insoluble in water
Solubility
Solubility can be defined as:
a)
the relative ability of a solute to form a solution
when added to a certain solvent
b)
the maximum amount of solute you can dissolve in a
fixed amount of solvent at a given temperature
Forming
a Solution
To form a solution, the solute particles must be attracted to the
solvent particles; e.g., salt particles are attracted to water particles
We can say that salt is soluble
in water because it dissolves in water
Salt and olive oil will not form a solution because the salt particles
are not attracted to the oil particles
We can say that salt is insoluble
in olive oil because it does not dissolve in olive oil
Concentration – Qualitative
A concentrated solution: A
solution that contains a lot of dissolved solute compared to the amount of
solvent; e.g., a can of frozen orange juice
A dilute solution: A solution
that contains very little solute compared to the amount of solvent; e.g., a
solution of water and the can of frozen orange juice
Concentration – Quantitative
The concentration of a solution can be written as the amount of solute
dissolved in a specific amount of solvent.
For example, if 5 grams of salt are dissolved in 500 milliliters of
water; the concentration is 5g/500mL
Often we reduce this to a value out of 100mL; so it can be written as 1
gram/100mL
This can also be called a 1 percent solution; this means that for 100mL
of solvent there is 1g of solute dissolved in it
Saturation
Saturation: The maximum
amount of solute that can be dissolved in a certain amount of solvent at a
certain temperature
Saturated Solution: one that has been
saturated; no more solute can be dissolved
Saturation Point: The point at which
no more solute can be dissolved in a fixed volume of solvent at that
temperature
If more solute can be dissolved into a solvent at a given temperature,
then it is called an unsaturated
solution.
Sometimes a saturated solution can be cooled below a critical
temperature to form a supersaturated
solution. This type of solution contains more solute that would normally be
dissolved in the solution.
Tuesday 9 February 2016
Monday 8 February 2016
Wednesday 3 February 2016
Review Activities for Chapter 7 Test
Chapter 7.1 Quiz
http://www.sciencesource.ca/quizzes/quiz_07_1BOmTe.htm
Chapter 7.2 Quiz
http://www.sciencesource.ca/quizzes/quiz_07_2b3Xn9.htm
Chapter 7 Quiz
http://wps.pearsoned.ca/ca_school_ontarioscience_7-8/102/26140/6692048.cw/content/index.html
Chapter Labeling Practice
http://www.sciencesource.ca/resources/gr7_matchquiz_ch07/
Chapter Matching Practice
http://www.sciencesource.ca/resources/gr7_matchquiz_ch07/
Chapter 7.1 Crossword
https://sciencesource.pearsoncanada.ca/puzzles/puzzle_07_1/
Chapter 7.2 Crossword
https://sciencesource.pearsoncanada.ca/puzzles/puzzle_07_2/
Tuesday 2 February 2016
Section 7.1 Notes
Classification of Matter by Composition
Matter – anything that has mass and takes up space
Mass – the amount of matter in the object (grams, Kg)
Volume - the amount of space that matter occupies (m3, mL)
Solid - matter that has a definite shape and volume.
Particles are vibrating in place, but they cannot move freely.
Liquid – matter that does not have a definite shape but does have a definite volume; a liquid takes the shape of its container.
Particles can move freely past each other, however, they remain in a fixed volume.
A gas is matter that does not have a definite shape or volume.
Gas particles can move freely and fill the spaces they are in.
Pure substance – a substance made up of only one type of matter; e.g., sugar, distilled water, copper wire are all pure substances. Pure substances appear uniform or homogeneous; this means that every part of the substance has the same composition as every other part.
Mixture – a substance made up of two or more different substances; e.g., pizza, soft drinks, eggs. Each substance inside the mixture keeps its own properties; e.g., you can’t see the sugar in soft drinks, but you can taste it.
Classifying Mixtures – mixtures can be grouped into 2 major categories; mechanical mixtures and solutions.
Mechanical mixtures – the different parts of the mixture can be seen; the mixture does not have the same properties throughout.
Sometimes, it’s easy to see the different types of matter throughout the mixture; e.g., snack mix; sometimes you need a microscope.
These mixtures are said to be heterogeneous, this means that it is made up of different substances with different appearances and properties.
Solutions – these mixtures have the same appearance throughout, but they are made up of two or more substances. Solutions are made when you dissolve one substance into another.
All solutions are said to be homogeneous mixtures because they look the same throughout even though they are made up of different substances; e.g., dissolving sugar in tea.
Friday 29 January 2016
Chapter 7 Test Study Guide
Here is the study guide for your upcoming Chapter 7 Test on Friday February 5th, 2016
- Classifying mixtures:
- pure substances and mixtures
- heterogeneous mixtures (mechanical mixtures) and homogeneous mixtures (solutions)
- Provide examples of these substances
- Review the 6 points of the particle theory of matter
- Review the behaviour of particles in solids liquids and gases
- Review the 6 changes of state and the diagram in the package; review which changes of state require energy and which require energy to be removed to occur
- Understand how and why substances change state according the the particle theory
- Review the difference between heat and temperature
Key Terms
- Matter
- Pure Substance
- Solid, liquid, gas
- Mixture
- Homogeneous
- Heterogeneous
- Homogeneous mixture (Solution)
- Heterogeneous mixture (Mechanical mixture
- The particle theory of matter
- Temperature
- Heat
- Change of state
- Evaporation
- Boiling
- Condensation
- Melting
- Solidification (freezing)
- Sublimation
- Deposition
Thursday 28 January 2016
Wednesday 27 January 2016
Three States of Matter Notes
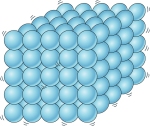
SOLID
- Solids have very low kinetic energy (the energy of movement)
- The particles vibrate in place
- Solids have a definite shape and volume (they cannot be compressed)
- The particles are very close together
- The particle attraction is very strong
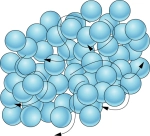
LIQUID
- Liquids have high kinetic energy
- The particles are able to move past each other
- Liquids take the shape of the container they are in (they do not have a definite shape)
- Liquids have a definite volume (they cannot be compressed into a smaller space)
- There are spaces between the particles
- The particles have low particle attraction
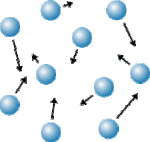
GAS
- Gases have very high kinetic energy
- Gas particles move freely
- The particles fill the space they are in (they do not have a definite shape or volume)
- Gases CAN be compressed into smaller spaces
- There are very large spaces between the particles
- The particles have very low particle attraction
*** Kinetic energy increases as you move from SOLIDS to GASES
*** Particle attraction decreases as you move from SOLIDS to GASES
Tuesday 26 January 2016
Monday 25 January 2016
Classifying Materials Worksheet Answers
|
Material
|
Heterogeneous or
homogeneous
|
Reason
|
|
Honey
|
Homogeneous
|
If there are no bubbles, all parts of the honey look the
same
|
|
Yogurt with fruit
|
Heterogeneous
|
We see the yogurt and the fruit separately
|
|
Marshmallow
|
Heterogeneous
|
The inside is sticky and the outside is dry and powdery
|
|
Raisin bread
|
Heterogeneous
|
There are raisins and the bread which can be easily
identified
|
|
Orange juice with pulp
|
Heterogeneous
|
We can see solid pulp and liquid orange juice
|
|
Vanilla ice cream
|
Homogeneous
|
All parts of the vanilla ice cream look the same
|
|
Concrete sidewalk
|
Heterogeneous
|
Contains many different small rocks
|
|
Plastic cup
|
Homogeneous or heterogeneous
|
Depending on the cup, there may be different colours or
textures
|
|
Ketchup
|
Heterogeneous
|
The liquid portion separates from the solid portion
|
|
Sand
|
Heterogeneous
|
Sand is made up of crushed rocks or different colours and
materials
|
Subscribe to:
Posts (Atom)
