Classification of Matter by Composition
Matter – anything that has mass and takes up space
Mass – the amount of matter in the object (grams, Kg)
Volume - the amount of space that matter occupies (m3, mL)
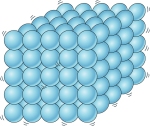
Solid - matter that has a definite shape and volume.
Particles are vibrating in place, but they cannot move freely.
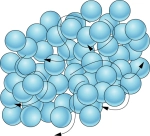
Liquid – matter that does not have a definite shape but does have a definite volume; a liquid takes the shape of its container.
Particles can move freely past each other, however, they remain in a fixed volume.
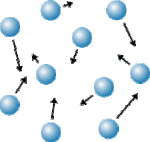
A gas is matter that does not have a definite shape or volume.
Gas particles can move freely and fill the spaces they are in.
Pure substance – a substance made up of only one type of matter; e.g., sugar, distilled water, copper wire are all pure substances. Pure substances appear uniform or homogeneous; this means that every part of the substance has the same composition as every other part.
Mixture – a substance made up of two or more different substances; e.g., pizza, soft drinks, eggs. Each substance inside the mixture keeps its own properties; e.g., you can’t see the sugar in soft drinks, but you can taste it.
Classifying Mixtures – mixtures can be grouped into 2 major categories; mechanical mixtures and solutions.
Mechanical mixtures – the different parts of the mixture can be seen; the mixture does not have the same properties throughout.
Sometimes, it’s easy to see the different types of matter throughout the mixture; e.g., snack mix; sometimes you need a microscope.
These mixtures are said to be heterogeneous, this means that it is made up of different substances with different appearances and properties.
Solutions – these mixtures have the same appearance throughout, but they are made up of two or more substances. Solutions are made when you dissolve one substance into another.
All solutions are said to be homogeneous mixtures because they look the same throughout even though they are made up of different substances; e.g., dissolving sugar in tea.